-

Online glove integrity testing: the new industry standard and paradigm shift for pharmaceutical cleanroom glove integrity testting
Glove integrity is a primary control point for aseptic processing: isolator and RABS gloves form the physical barrier protecting sterile product from contamination. Recent regulatory updates — notably EMA Annex 1 (revised 2023) — and increasing inspection emphasis on data integrity mean manufacturers must reassess legacy approaches. Industry surveys and vendor case studies report test-cycle time reductions from several hours to under 10 minutes per glove when using online systems, enabling more frequent checks without production loss (see regulatory and business sections below for sources).MORE -

2025 ISPE Annual Meeting & Expo,October 27-29
Welcome visit us on 2025 |SPE Annual meeting, our boothis 1312.MORE -

Exhibition Success Report,The 67th (2025) China International Pharmaceutical Machinery Exposition (CIPM) in Qingdao
Over the three-day Qingdao Pharma Machinery Expo, Biodeconta not only comprehensively demonstrated its technical strength in the fields of sterile testing, spatial sterilization and laboratory equipment, but also accurately identified industry pain points and market trends through in-depth face-to-face exchanges.MORE -

Biodeconta Shines at the 2025 PDI Conference
Biodeconta delivered an impressive performance at this conference. Numerous customers showed strong interest in its glove leak detectors, stopping by the booth one after another to inquire in detail about the product performance, operation methods, and maintenance key points. They recognized the product’s crucial role in ensuring production safety and spoke highly of its precise detection capabilities.MORE -

Review the Chongqing Pharmaceutical Machinery Exhibition
At the recently held Pharmaceutical Machinery Exhibition, BIODECONTA shone brightly in Hall C1 on the international stage. Despite the varying levels of foot traffic in different parts of the exhibition hall, BIODECONTA attracted numerous customers to stop by with its meticulously designed booth and high-quality core products, becoming a major highlight of the exhibition.MORE -

《Interpretation of Standard Practice for Quality Risk Management in Aseptic Processing》
The Parenteral Drug Association (PDA) is a globally recognized authoritative organization in the pharmaceutical industry, and its established standards provide a scientifically rigorous quality management system for aseptic drug manufacturing.As the latest guideline, PDA/ANSI 03-2025 Standard Practice for Quality Risk Management in Aseptic Processing explicitly introduces the concept of "full lifecycle contamination control," emphasizing end-to-end management from contamination source identification and risk control to continuous improvement.MORE -

INTERPHEX 2025
"Join BIODECONTA at INTERPHEX 2024 – Booth #3638!
We’re excited to showcase our glove leak detector (GISTOOL) and hydrogen peroxide generators, along with our decontamination services.
As a trusted innovator in biodecontamination and critical environment control, BIODECONTA has been empowering pharmaceutical, healthcare, and life science industries with cutting-edge contamination prevention solutions since 2016. Our mission is to ensure the highest standards of safety and compliance through precision-engineered products and expert sterilization services.MORE -

PDA Week
BIODECONTA Cordially Invites You to Visit Booth #116 at PDA Week in Palm Springs, CA (April 7-9)
Discover our cutting-edge GISTOOL Glove Leak Detector, Hydrogen Peroxide Generators, and Decontamination Solutions as we explore industry innovations together.MORE -

Biodeconta Shines at Xiamen Pharmaceutical Machinery Exhibition, and International Cooperation Ushers in a New Journey for the Pharmaceutical Industry
From November 17th to 19th, 2024, Biodeconta shone brightly at the China Pharmaceutical Equipment Exhibition.
At the exhibition site, the Biodeconta booth had a unique feature. The main color scheme of blue and white was bright, elegant, and in line with the characteristics of the pharmaceutical industry. The smooth layout design guided people to explore in depth.MORE -
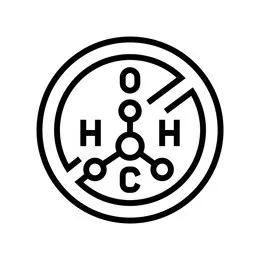
Do you know about hydrogen peroxide?
Strong sterilization ability: It can kill bacteria, fungi, viruses, spores and highly resistant bacterial spores (hydrogen peroxide fumigation can reach the level of LG6 sanitize, so that the number of microorganisms can be reduced by 6 logs, such as n*106→n, and the microbial killing rate is 99.9999%. )。MORE -

Sterilization? Cleanse? Silly and can't tell the difference?
Do "space purification" and "space sterilization" mean the same thing to you? In the editor's view, they all play a role in sanitizing the space. If you think the same as the editor, then you are very wrong! In order to let you truly understand the meaning of "sterilization" and "purification", we have invited Marc Xiong, Executive Director of BiodecontaMORE -

Biodeconta origins
Biodeconta is a dream that belongs to every Biodeconta person who wants to be able to provide truly efficient and high-quality microbial purification services, and to every Biodeconta who wants to have truly safe and intelligent microbial decontamination servicesMORE

